Optical Manipulation
Characterisation Installation 5
The aim of the Optical Manipulation (OM)-Lab is to develop new optical manipulation instrumentation and techniques for nanotechnology, biophysics and biomedicine and apply them to cell biology experiments.
At present OM-Lab runs optical manipulation stations including multiple trapping, manipulation, force measurements, laser microsurgery, Raman spectroscopy, phase contrast imaging, TIRF and FRET.
Local probing the mechanical properties of living cells and local stimulation of specific compartments of neuronal cells with a small amount of neuronal activation molecules represent two main directions of our research activity. Local probing of the mechanical properties employs optical tweezers (OT) force spectroscopy to measure forces exerted by membrane tether membranes on a pulling bead and derive local viscoelastic properties of the cell. OT are used also to locally measure cell elasticity by indentation method similarly to AFM but with different loading characteristics.
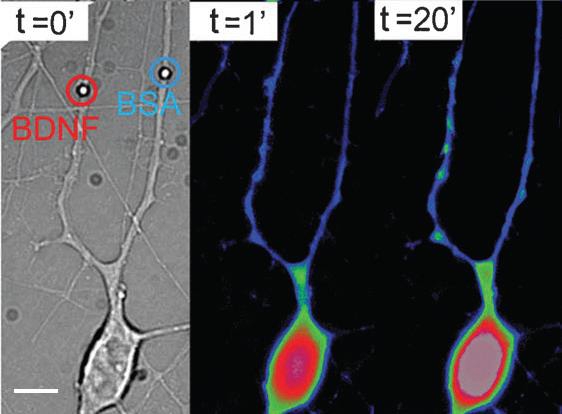
These methods are applied at OM-Lab to study the mechanical properties of cancer cells characterized by different levels of disease aggressiveness. On the other hand, digital holographic and speckle microscopies are employed to measure the thermal vibration of suspended cells, like red blood cells, and establish protocols to detect cell infection, e.g. malaria. Local stimulation of the neuronal cells has as goal to mimic biomechanical and biochemical interactions between hippocampal neurons, in order to understand synaptic and development mechanisms. These activities are developed in collaboration with SISSA and University of Trieste. Biomechanical interactions are mimicked using optically manipulated beads positioned in front of processes and measuring interaction forces developed there. Micro- and nano-vectors coated or filled with active molecules are instead used to stimulate specific compartments of the neurons. Examples of vectors are: microbeads, quantum dots, PLGA biodegradable beads, liposomes, micro-vesicles released by cells. The vectors are trapped and positioned on cells by optical tweezers microscopy. Stimulation is reached by cell-vector contact (coated beads or QDs), photolysis (liposomes) or by biodegradation (PLGA beads). The effects induced are observed by optical microscopy, following the cell morphology or/and activation of specific process indicators in the cell. An example of neuronal stimulation with Brain Derived Neurothrophic Factor (BDNF) is shown in the figure above: Brightfield (left) and Ca ++ fluorescence images (right) of a hippocampal neuron stimulated by two micrometric beads coated with BDNF and respectively Bovine Serum Albumin (BSA). The beads are precisely positioned on two different dendrites. BDNF coated bead induces an increase of the Ca ++ level in the corresponding dendrite and in the cell body after 20’.
https://www.iom.cnr.it/optical-manipulation-laboratory